5-amino-1mq 5mg
$50.00 - $65.00
You save
- Physical profile: Lyophilized powder
- This product is sold as a research chemical and not for human or animal consumption. For laboratory use by qualified professionals.
Availability: Ships today if ordered and paid by 12 PM EST. (Except Saturdays & Sundays)
Product Usage
This PRODUCT IS INTENDED AS A RESEARCH CHEMICAL ONLY. This designation allows the use of research chemicals strictly for in vitro testing and laboratory experimentation only. All product information available on this website is for educational purposes only. Bodily introduction of any kind into humans or animals is strictly forbidden by law. This product should only be handled by licensed, qualified professionals. This product is not a drug, food, or cosmetic and may not be misbranded, misused or mislabled as a drug, food or cosmetic.
5-Amino-1-methylquinolinium (5-Amino-1MQ) is a small molecule derivative of 1-methylquinolinium that selectively inhibits nicotinamide N-methyltransferase (NNMT), an enzyme involved in metabolic regulation and epigenetic control. By suppressing NNMT activity, 5-Amino-1MQ has shown potential in enhancing energy metabolism, reducing fat accumulation, and modulating cellular function in various preclinical models.
Introduction
5-Amino-1-methylquinolinium (5-Amino-1MQ) is an emerging compound of interest in metabolic and aging-related research due to its role as an inhibitor of the enzyme nicotinamide N-methyltransferase (NNMT). NNMT plays a crucial part in cellular metabolism and is upregulated in various metabolic disorders, including obesity, type 2 diabetes, and certain cancers. Elevated NNMT activity has been associated with decreased NAD+ availability and disrupted methylation balance, both of which are key factors in cellular aging and metabolic decline. By inhibiting NNMT, 5-Amino-1MQ can help restore NAD+ levels and methyl donor availability, thereby promoting more efficient cellular function and potentially reversing some age- or disease-related metabolic dysfunctions.
Research into 5-Amino-1MQ is still in its early stages, but preliminary studies in cell cultures and animal models have shown promising outcomes, including reduced adiposity, enhanced mitochondrial activity, and improved insulin sensitivity. Its potential benefits extend to muscle performance, fat metabolism, and longevity support. With its mechanism centered on rebalancing cellular bioenergetics and gene expression pathways, 5-Amino-1MQ offers a novel therapeutic angle for a wide range of metabolic and age-related conditions. This makes it a compelling subject for continued biomedical investigation and therapeutic development.
Overview
5-Amino-1-methylquinolinium (5-Amino-1MQ) is attracting significant interest for its biological effects that stem from its targeted inhibition of nicotinamide N-methyltransferase (NNMT). NNMT is an enzyme that catalyzes the methylation of nicotinamide using S-adenosylmethionine (SAM) as a methyl donor. This process generates 1-methylnicotinamide (1-MNA), reducing both nicotinamide (a precursor of NAD+) and SAM (a crucial methyl donor), leading to downstream consequences on cellular metabolism and epigenetic regulation. In many chronic metabolic diseases and cancers, NNMT is overexpressed, which contributes to pathological alterations in cellular energy balance and methylation patterns.
5-Amino-1MQ, by inhibiting NNMT, has a dual effect on metabolism. First, it helps preserve intracellular nicotinamide levels, thereby supporting NAD+ biosynthesis. NAD+ is essential for the function of sirtuins and other metabolic enzymes involved in mitochondrial health and oxidative metabolism. Second, it conserves methyl groups by reducing the consumption of SAM, which has implications for DNA and histone methylation, key mechanisms in gene expression regulation. Through these biochemical actions, 5-Amino-1MQ can influence both energy metabolism and epigenetic stability.
Preclinical studies involving 5-Amino-1MQ have yielded several promising results. In mouse models, administration of the compound has led to significant reductions in white adipose tissue without causing muscle loss. This suggests a preferential targeting of fat metabolism rather than general catabolism. Enhanced mitochondrial function has also been observed, correlating with increased expression of genes involved in oxidative phosphorylation and thermogenesis. Furthermore, treated animals exhibited improved glucose handling and insulin sensitivity, highlighting its therapeutic potential in metabolic syndrome and type 2 diabetes.
Beyond its metabolic effects, the epigenetic modulation driven by 5-Amino-1MQ is a subject of growing interest. By maintaining SAM pools and limiting aberrant methylation, the compound may stabilize the expression of genes that protect against inflammation, oxidative stress, and cellular senescence. This positions 5-Amino-1MQ not only as a metabolic modulator but also as a candidate for age-related disease intervention. There is also emerging interest in its potential neuroprotective effects, as NNMT activity is implicated in neurodegenerative disorders like Parkinson’s disease.
Pharmacologically, 5-Amino-1MQ is characterized by its small size and favorable cell permeability, making it a suitable candidate for oral or injectable formulations. However, data on its pharmacokinetics, toxicity profile, and long-term effects in humans are still lacking. Early-phase research continues to focus on mechanism elucidation and efficacy validation in animal models.
Overall, 5-Amino-1MQ represents a novel class of NNMT inhibitors with the potential to impact multiple physiological pathways. Its action at the intersection of metabolism and epigenetics provides a unique approach to treating complex diseases driven by energy imbalance and gene dysregulation. As research advances, the compound could find utility in therapeutic strategies aimed at obesity, diabetes, aging, and related chronic conditions.
Mechanism of action
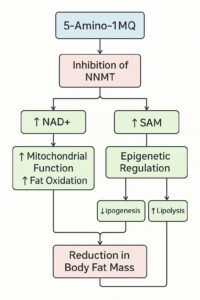
It works primarily by inhibiting the enzyme nicotinamide N-methyltransferase (NNMT), which plays a central role in both energy metabolism and epigenetic regulation. Here’s how it exerts its effects at the molecular level:
1. Inhibition of NNMT Activity
NNMT catalyzes the methylation of nicotinamide (NAM) using S-adenosylmethionine (SAM) as the methyl donor, producing 1-methylnicotinamide (1-MNA) and S-adenosylhomocysteine (SAH). This reaction consumes both NAM and SAM, depleting precursors that are critical for two essential pathways:
- NAD+ biosynthesis (from NAM)
- Methylation reactions (dependent on SAM)
By inhibiting NNMT, 5-Amino-1MQ:
- Reduces the conversion of NAM into 1-MNA
- Preserves NAM for NAD+ biosynthesis
- Prevents excessive SAM depletion, stabilizing methyl group availability for epigenetic and metabolic functions
2. Restoration of NAD+ Levels
Nicotinamide is a vital precursor for NAD+ (nicotinamide adenine dinucleotide), a coenzyme required by several cellular enzymes, especially sirtuins, PARPs, and CD38. These enzymes regulate:
- Mitochondrial function
- DNA repair
- Oxidative stress response
- Inflammatory signaling
By conserving nicotinamide, 5-Amino-1MQ indirectly increases NAD+ levels, which enhances:
- Mitochondrial biogenesis
- Fat oxidation
- Cellular energy production
3. Preservation of Methyl Donors
The SAM-to-SAH ratio is critical for DNA and histone methylation. When NNMT is overactive, SAM is excessively consumed, leading to a reduced methylation capacity and epigenetic instability. 5-Amino-1MQ helps maintain:
- SAM levels, ensuring normal methylation patterns
- Epigenetic balance, supporting healthy gene expression
This contributes to:
- Reduced inflammation
- Controlled cell proliferation
- Protection against age-related gene dysregulation
4. Downregulation of NNMT-Driven Pathologies
NNMT overexpression is associated with:
- Obesity (by stabilizing fat storage gene expression)
- Insulin resistance
- Cancer (via epigenetic silencing of tumor suppressor genes)
By inhibiting NNMT, 5-Amino-1MQ counteracts these pathological effects, promoting:
- Fat loss
- Improved insulin sensitivity
- Anti-tumor and anti-aging effects (in preclinical models)
In summary, 5-Amino-1MQ’s mechanism of action centers on blocking NNMT to rebalance NAD+ and SAM levels, thereby enhancing mitochondrial function, supporting epigenetic stability, and modulating metabolism and inflammation.
Structure
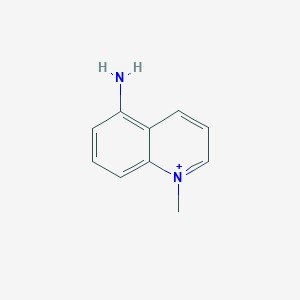
Chemical Formula: C10H11N2+
Molecular Weight: 159.21 g/mol
PubChem CID: 950107
CAS No.: 42464-96-0
Synonyms: 5-Amino-1-methylquinolinium, 1-Methyl-5-aminoquinolinium, 5-Amino-1-methylquinoline cation, NSC 122186 (research code)
Research
Role in obesity management
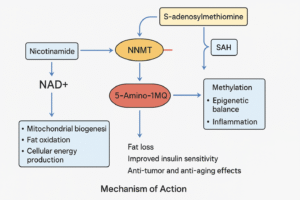
5-Amino-1-methylquinolinium (5-Amino-1MQ) has shown promise as a potential therapeutic agent in the management of obesity due to its ability to target and inhibit nicotinamide N-methyltransferase (NNMT), a metabolic enzyme implicated in fat storage and energy expenditure. NNMT is upregulated in white adipose tissue in obese individuals and plays a key role in the regulation of cellular energy metabolism and methylation status. Elevated NNMT activity results in the depletion of nicotinamide and S-adenosylmethionine (SAM), disrupting NAD+ biosynthesis and methylation processes essential for metabolic balance (Kraus D, 2014).
By inhibiting NNMT, 5-Amino-1MQ helps restore NAD+ levels, which in turn enhances mitochondrial function and fat oxidation. Increased NAD+ activates sirtuins, particularly SIRT1, which is known to regulate genes involved in energy homeostasis, lipolysis, and thermogenesis (Parsons RB, 2013). Moreover, the conservation of SAM supports proper DNA and histone methylation, allowing favorable expression of genes involved in lipid metabolism and insulin sensitivity.
Preclinical studies in mice have shown that administration of 5-Amino-1MQ leads to a significant reduction in body fat mass without compromising lean muscle mass. For instance, inhibiting NNMT in white adipose tissue using small molecule inhibitors (like 5-Amino-1MQ analogs) increased energy expenditure and reduced weight gain in diet-induced obese mice, independent of food intake (Kraus D, 2014).
Additionally, 5-Amino-1MQ improves glucose tolerance and insulin sensitivity, making it beneficial not only for weight loss but also for mitigating metabolic complications such as insulin resistance and type 2 diabetes that often accompany obesity (Parsons RB, 2013).
Overall, 5-Amino-1MQ functions by reprogramming metabolic pathways at the cellular level, turning energy-storing white adipose tissue into a more energy-expending phenotype, often described as “browning” of fat. This makes it a novel and mechanistically unique candidate for obesity management (Kraus D, 2014; Parsons RB, 2013).
Here is flow chart showing its therapeutic role in management of obesity.
Role in muscle function
Beyond its metabolic benefits in adipose tissue, 5-Amino-1MQ also appears to play a supportive role in muscle function by preserving mitochondrial integrity, enhancing energy production, and improving insulin signaling within skeletal muscle cells. These effects are largely indirect, stemming from the compound’s ability to inhibit nicotinamide N-methyltransferase (NNMT), a regulatory enzyme that consumes nicotinamide and depletes methyl donor pools (Kraus D, 2014).
In muscle tissue, NAD+ availability is critical for the function of sirtuins, especially SIRT1 and SIRT3, which regulate mitochondrial biogenesis, oxidative metabolism, and cellular stress responses. When NNMT is overexpressed—as seen in aging and insulin-resistant states—NAD+ levels drop, leading to impaired mitochondrial activity and reduced ATP production. By inhibiting NNMT, 5-Amino-1MQ helps preserve intracellular nicotinamide, thus supporting NAD+ synthesis and enhancing mitochondrial function (Hong S, 2015).
In a study by Kraus et al., NNMT inhibition in obese mice not only reduced fat mass but also spared lean muscle mass, suggesting that the compound does not induce general catabolism, a common side effect of many weight-loss agents (Kraus D, 2014). Additionally, improved glucose uptake and insulin sensitivity in skeletal muscle was observed, pointing to enhanced metabolic flexibility and muscle efficiency (Parsons RB, 2013).
Furthermore, the maintenance of SAM levels through NNMT inhibition is important for epigenetic regulation of genes involved in muscle regeneration, inflammation control, and oxidative stress resistance. These effects may translate into improved muscle recovery, reduced fatigue, and better performance, although human studies are still lacking and most data come from preclinical models (Ulanovskaya OA, 2013).
Collectively, 5-Amino-1MQ supports muscle function by:
- Preserving NAD+ levels → enhanced mitochondrial efficiency and ATP production
- Improving insulin sensitivity → better glucose uptake by muscle cells
- Maintaining SAM availability → stabilizing gene expression linked to muscle health
- Sparing muscle mass during fat loss interventions
These properties make 5-Amino-1MQ a promising candidate for conditions associated with muscle decline, such as aging (sarcopenia), metabolic syndrome, and recovery from immobilization or chronic illness (Kraus D, 2014; Hong S, 2015).
Possible role in cognition
While direct human studies on 5-Amino-1MQ and cognition are limited, emerging research suggests that its inhibition of nicotinamide N-methyltransferase (NNMT) may exert neuroprotective and cognition-enhancing effects, largely through its impact on NAD⁺ metabolism, mitochondrial function, and epigenetic regulation.
NNMT is increasingly recognized as a regulator of neuronal energy balance and oxidative stress. Overexpression of NNMT in the brain has been associated with NAD⁺ depletion, mitochondrial dysfunction, and accumulation of neurotoxic byproducts, all of which can contribute to cognitive decline and neurodegeneration (Ulanovskaya OA, 2013). 5-Amino-1MQ, by inhibiting NNMT, helps preserve intracellular nicotinamide, the precursor for NAD⁺, thereby enhancing NAD⁺-dependent processes critical to brain function.
Increased NAD⁺ levels activate sirtuins, particularly SIRT1 and SIRT3, which modulate neuroinflammation, mitochondrial biogenesis, and DNA repair — mechanisms essential for maintaining cognitive health and slowing aging-related neuronal decline (Verdin E, 2015). SIRT1, for instance, deacetylates transcription factors such as PGC-1α and FOXO, promoting antioxidant defenses and reducing oxidative damage in neurons (Donmez G, 2011).
Moreover, SAM preservation through NNMT inhibition supports proper DNA and histone methylation, crucial for learning, memory formation, and synaptic plasticity. Disruption of methylation homeostasis has been linked to cognitive impairment and disorders such as Alzheimer’s disease (Fuso A, 2012). By supporting balanced methylation, 5-Amino-1MQ may help maintain the epigenetic integrity of cognitive networks.
Although most current findings are preclinical, they are promising. For instance, increased NNMT expression has been observed in brains affected by neurodegenerative diseases like Parkinson’s and Alzheimer’s (Aksoy S, 1994), and targeting NNMT has been proposed as a potential intervention to restore redox balance and energy metabolism in aging neurons.
In summary, 5-Amino-1MQ may support cognitive function by:
- Enhancing NAD⁺ levels → supports sirtuin activity and mitochondrial health
- Reducing oxidative stress and inflammation in the brain
- Stabilizing epigenetic regulation essential for memory and neuroplasticity
- Potentially slowing cognitive decline in age-related or neurodegenerative conditions
While clinical trials are needed, these molecular pathways provide a strong rationale for investigating 5-Amino-1MQ as a neurotherapeutic candidate.
References
- Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508(7495):258-62. doi:10.1038/nature13198.
- Parsons RB, Smith ML, Williams AC, Waring RH, Ramsden DB. High expression of nicotinamide N-methyltransferase in patients with obesity and insulin resistance. Diabetes Obes Metab. 2013;15(2):137–145. doi:10.1111/j.1463-1326.2012.01585.x..
- Hong S, Moreno-Navarrete JM, Wei X, Kikukawa Y, Tzameli I, Prasad D, et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med. 2015;21(8):887–894. doi:10.1038/nm.3882.
- Parsons RB, Smith ML, Williams AC, Waring RH, Ramsden DB. High expression of nicotinamide N-methyltransferase in patients with obesity and insulin resistance. Diabetes Obes Metab. 2013;15(2):137–145. doi:10.1111/j.1463-1326.2012.01585.x.
- Ulanovskaya OA, Zuhl AM, Cravatt BF. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol. 2013;9(5):300–306. doi:10.1038/nchembio.1204.
- Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–1213. doi:10.1126/science.aac4854.
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142(2):320–332. doi:10.1016/j.cell.2010.06.020.
- Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D’Anselmi F, Coluccia P, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloidogenic processing in mice. Mol Cell Neurosci. 2008;37(4):731–746. doi:10.1016/j.mcn.2007.12.006.
- Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269(20):14835–14840.
Related products
-
Sale!
 Out of stock
Out of stock




