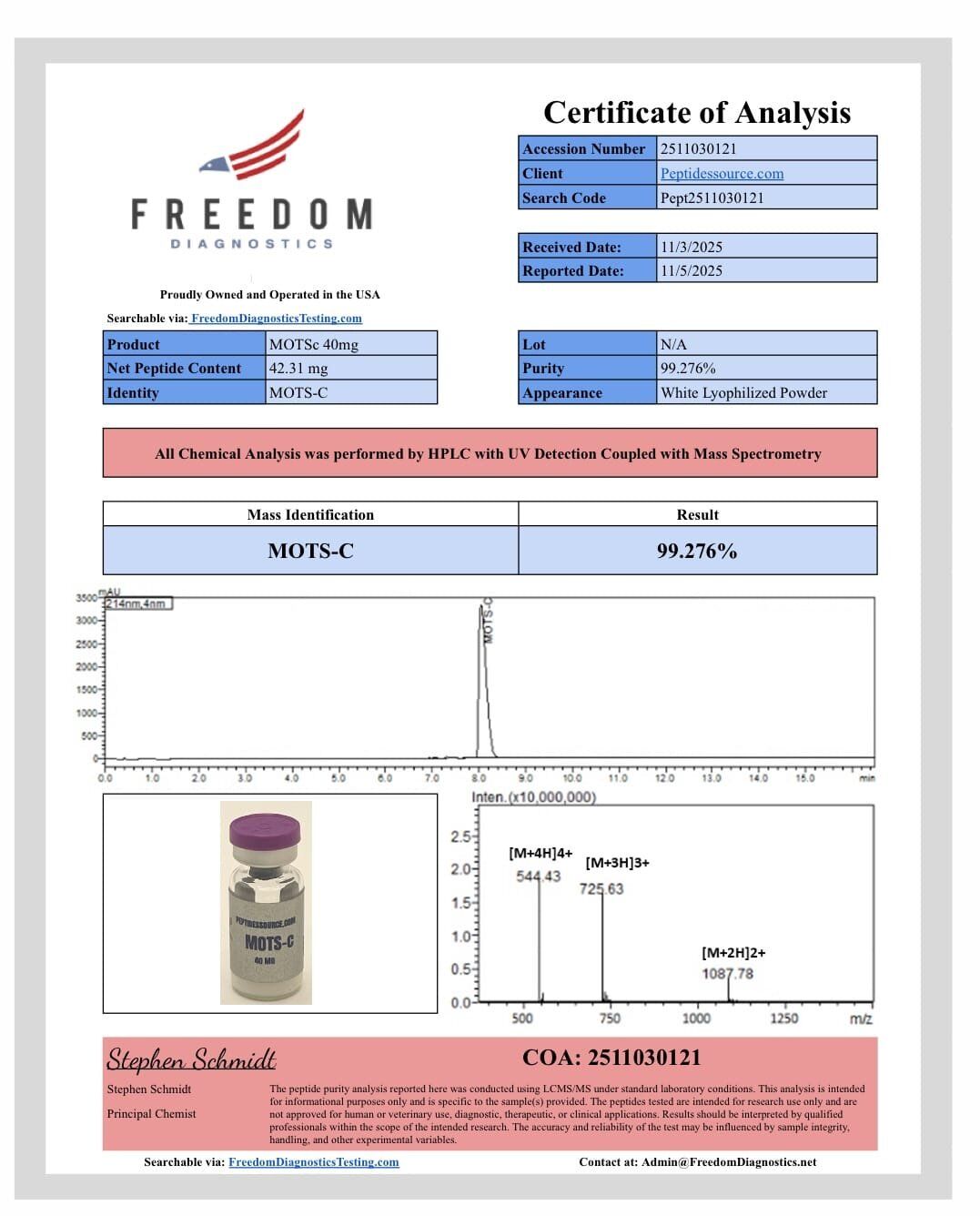
MOTS-C 40mg
$190.00
You save
- Physical profile: Lyophilized powder
- This product is sold as a research chemical and not for human or animal consumption. For laboratory use by qualified professionals.
Availability: 79 in stock (can be backordered)
Availability: Ships today if ordered and paid by 12 PM EST. (Except Saturdays & Sundays)
Product Usage
MOTS-C 40mg IS INTENDED AS A RESEARCH CHEMICAL ONLY. This designation allows the use of research chemicals strictly for in vitro testing and laboratory experimentation only. All product information available on this website is for educational purposes only. Bodily introduction of any kind into humans or animals is strictly forbidden by law. This product should only be handled by licensed, qualified professionals. This product is not a drug, food, or cosmetic and may not be misbranded, misused or mislabled as a drug, food or cosmetic.
Overview
MOTS-c is a mitochondrial-derived peptide that exemplifies the emerging concept of mitochondrial-nuclear communication via bioactive peptides. It is composed of 16 amino acids and encoded by a short open reading frame (sORF) within the mitochondrial 12S rRNA gene, which is highly conserved across species. Unlike traditional nuclear-encoded peptides, MOTS-c is translated from mitochondrial RNA and participates in cellular signaling pathways that extend well beyond the mitochondria. One of the hallmark features of MOTS-c is its ability to translocate to the nucleus in response to metabolic stress, such as glucose restriction or oxidative stress. Upon nuclear entry, it modulates the expression of genes associated with metabolism, stress resistance, and cell survival. This process involves interaction with transcription factors like NRF2 and AMPK-related signaling pathways, which are central to cellular adaptation under energy-deprived conditions. MOTS-c plays a crucial role in glucose homeostasis by promoting glucose uptake and glycolysis, while simultaneously inhibiting the accumulation of fat. It enhances insulin sensitivity and reduces insulin resistance, making it a promising candidate for managing conditions like type 2 diabetes and metabolic syndrome. In rodent models, administration of MOTS-c led to improved glucose tolerance, reduced body weight, and increased energy expenditure. Its influence on muscle physiology is also significant. MOTS-c levels decline with age, and its supplementation has been shown to enhance skeletal muscle function and endurance in aged mice. This suggests a role in mitigating age-related sarcopenia and promoting mitochondrial biogenesis. Furthermore, by activating AMPK, MOTS-c mimics the effects of exercise at the molecular level, earning it the label of an “exercise mimetic” in some studies. In addition to its metabolic benefits, MOTS-c exhibits cytoprotective properties. It protects cells from oxidative damage and apoptosis through mitochondrial pathways and may contribute to lifespan extension under certain conditions. Recent research is exploring its neuroprotective potential, given that metabolic dysfunction and mitochondrial impairments are common features in neurodegenerative disorders. What distinguishes MOTS-c from other peptides is its systemic endocrine-like activity despite being of mitochondrial origin. It circulates in the bloodstream and exerts effects on distant tissues, indicating a hormonal function. Its ability to bridge mitochondrial signals to nuclear gene regulation places it at the intersection of metabolism, aging, and stress adaptation. Therapeutically, MOTS-c represents a promising avenue for treating metabolic disorders, age-related muscle loss, and possibly even cognitive decline. Synthetic analogs of MOTS-c are being explored for clinical development due to their stability and efficacy in preclinical trials. In conclusion, MOTS-c is not only a novel mitochondrial product but also a key regulatory molecule in systemic physiology. Its discovery has redefined mitochondrial biology and opened new strategies for targeting metabolic and age-associated diseases. 
Mechanism of action
MOTS-c (Mitochondrial ORF of the 12S rRNA type-c) exerts its physiological effects through multi-level metabolic regulation, integrating mitochondrial signaling, nuclear gene modulation, and cellular stress response pathways. Its mechanism of action involves the following key steps:1. Mitochondrial Stress Sensing and Cytoplasmic Release
MOTS-c is encoded by a small open reading frame (sORF) within the mitochondrial 12S rRNA. Under conditions of metabolic stress (e.g. glucose deprivation, oxidative stress), MOTS-c is synthesized within the mitochondria and translocates into the cytoplasm, initiating downstream signaling cascades.2. Nuclear Translocation and Gene Regulation
Upon cellular energy stress, MOTS-c moves from the cytoplasm to the nucleus, where it interacts with nuclear transcription factors such as NRF2 (Nuclear Factor Erythroid 2–related Factor 2). This interaction enhances the transcription of antioxidant response genes and metabolic genes involved in glucose metabolism, mitochondrial function, and stress resistance.3. Activation of AMPK Pathway
MOTS-c strongly activates AMP-activated protein kinase (AMPK), a central regulator of cellular energy balance. This leads to:- Increased glucose uptake by upregulating GLUT4 transporters
- Enhanced fatty acid oxidation
- Inhibition of anabolic pathways (e.g. mTOR) to conserve energy
- Improved mitochondrial biogenesis via PGC-1α activation
4. Inhibition of Folate Cycle to Regulate One-Carbon Metabolism
MOTS-c also inhibits the folate cycle by targeting methylenetetrahydrofolate dehydrogenase (MTHFD), which decreases de novo purine biosynthesis. This creates a mild purine depletion, activating AMPK as a secondary response. This novel mechanism links nucleotide metabolism with energy sensing.5. Systemic Endocrine-Like Effects
Although mitochondrial in origin, MOTS-c is found in the circulation, acting in an endocrine-like fashion. It influences distant tissues such as:- Muscle: Enhancing performance and insulin sensitivity
- Adipose tissue: Promoting fat oxidation
- Liver: Reducing gluconeogenesis and lipid accumulation
Summary Flow:
Mitochondrial stress → MOTS-c synthesis → Nuclear entry → AMPK activation → Metabolic gene regulation → Enhanced stress resilience and energy metabolismStructure
Aminoacid Sequence: Met-Arg-Trp-Gin-Glu-Met-Gly-Tyr-lle-Phe-Tyr-Pro-Arg-Lys-Leu-Arg Molecular Formula: C101 H152.N28O22,S2. Molecular Weight: 2174.64 g/mol PubChem SID: 255386757 CAS Number: 1627580-64-6 Synonyms: Mitochondrial open reading frame of the 12S RNA-c, MT-RNR1Research
Muscle metabolism
MOTS-c plays a significant role in regulating skeletal muscle metabolism, particularly under conditions of metabolic stress and aging. It influences key pathways associated with energy homeostasis, mitochondrial function, and exercise adaptation, thereby enhancing muscle function and resilience.- Activation of AMPK Pathway in Muscle MOTS-c activates AMP-activated protein kinase (AMPK), a crucial energy sensor in muscle cells. AMPK activation leads to:
- Increased glucose uptake via GLUT4 translocation
- Enhanced fatty acid oxidation
- Inhibition of anabolic pathways (e.g., mTOR), which promotes energy conservation during exercise or stress These effects contribute to improved muscle endurance, energy efficiency, and insulin sensitivity (Lee C et al., 2015).
- Enhancement of Mitochondrial Biogenesis MOTS-c induces PGC-1α, a master regulator of mitochondrial biogenesis, promoting the formation of new mitochondria and improving oxidative capacity in skeletal muscles. This supports better energy production and resistance to fatigue (Reynolds JC et al., 2021).
- Exercise Mimetic Effects MOTS-c mimics certain molecular aspects of physical exercise. In animal studies, administration of MOTS-c improved muscle performance and physical capacity without actual physical training, which is particularly beneficial for aging or immobilized individuals (Lu H et al., 2019).
- Prevention of Age-Related Muscle Decline (Sarcopenia) Age-related decline in MOTS-c levels correlates with muscle loss and weakness. Supplementation restores muscle metabolic flexibility and protects against sarcopenia in aged mice, possibly by improving protein turnover and enhancing insulin signaling in muscle tissues (Reynolds JC et al., 2021).
- Insulin Sensitivity and Glucose Metabolism In skeletal muscle, MOTS-c improves insulin sensitivity by enhancing glucose transport and reducing intramuscular fat accumulation, which is key for preventing insulin resistance and type 2 diabetes (Kim KH et al., 2018).
Fat metabolism
MOTS-c plays a critical role in fat metabolism by modulating energy balance, enhancing lipid utilization, and preventing fat accumulation. Its effects are mediated through mitochondrial signaling, activation of metabolic regulators, and endocrine-like functions across key tissues such as adipose tissue, liver, and skeletal muscle.1. Enhancement of Lipid Oxidation
MOTS-c stimulates fatty acid oxidation in multiple tissues by activating AMP-activated protein kinase (AMPK), a central energy-sensing enzyme. AMPK activation leads to:- Suppression of acetyl-CoA carboxylase (ACC) activity
- Reduction in malonyl-CoA, an inhibitor of carnitine palmitoyltransferase-1 (CPT1)
- Increased mitochondrial uptake and oxidation of fatty acids These processes reduce lipid accumulation and enhance overall lipid metabolism (Lee C et al., 2015).
2. Reduction of Adiposity and Lipid Storage
In animal studies, chronic MOTS-c treatment prevents diet-induced obesity by reducing fat mass without affecting food intake. This is partly due to increased thermogenic activity and energy expenditure, possibly through upregulation of uncoupling proteins (UCPs) in adipose tissue (Reynolds JC et al., 2021).3. Improvement of Insulin Sensitivity in Adipose Tissue
MOTS-c improves insulin signaling in adipocytes, facilitating glucose uptake and lipolysis. Enhanced insulin sensitivity reduces lipogenesis (fat creation) and promotes lipolysis (fat breakdown), further supporting anti-obesity effects (Kim KH et al., 2018).4. Regulation of Adipokines and Inflammatory Markers
MOTS-c modulates the expression of adipokines (e.g., adiponectin) and reduces inflammatory cytokines like TNF-α and IL-6, which are known to disrupt lipid metabolism and promote obesity. By creating a healthier adipose environment, MOTS-c supports better fat utilization and metabolic function (Lu H et al., 2019).5. Prevention of Hepatic Steatosis
MOTS-c also protects against fat accumulation in the liver (hepatic steatosis) by suppressing lipogenesis and promoting fat oxidation. This is crucial for preventing fatty liver disease, a common consequence of metabolic dysfunction (Reynolds JC et al., 2021).Insulin sensitivity
Role of MOTS-c in Insulin Sensitivity
It significantly enhances insulin sensitivity by modulating metabolic pathways, improving glucose homeostasis, and activating energy-regulating signals. Its insulin-sensitizing effects have been demonstrated in both cellular and animal models, particularly in the context of diet-induced obesity and insulin resistance.1. AMPK Activation and Glucose Uptake
It activates AMP-activated protein kinase (AMPK), a central regulator of cellular energy homeostasis. AMPK activation enhances:- Translocation of GLUT4 transporters to the cell membrane in skeletal muscle and adipose tissue
- Increased glucose uptake independent of insulin
- Improved insulin signaling efficiency, especially in insulin-resistant conditions (Lee C et al., 2015)
2. Suppression of Mitochondrial Stress and Inflammation
It reduces oxidative stress and pro-inflammatory cytokines (e.g., TNF-α, IL-6), both of which are known to impair insulin signaling. By maintaining mitochondrial integrity and reducing endoplasmic reticulum stress, MOTS-c helps restore normal insulin receptor activity and downstream signaling (Kim KH et al., 2018).3. Enhanced Insulin Sensitivity in Animal Models
In high-fat diet-fed mice, MOTS-c administration:- Improved insulin tolerance test (ITT) performance
- Reduced fasting insulin levels
- Enhanced glucose disposal rate
4. Nuclear Gene Regulation Linked to Insulin Pathways
MOTS-c translocates to the nucleus during metabolic stress and modulates transcription of genes involved in insulin signaling, including IRS-1, PI3K, and AKT pathways. This supports sustained insulin responsiveness under stress conditions (Kim KH et al., 2018).5. Synergy with Exercise and Caloric Restriction Pathways
It also mimics exercise-induced molecular changes,notably the activation of AMPK and PGC-1α, both of which enhance insulin sensitivity. This suggests its potential as a therapeutic mimetic of exercise for metabolic disorders (Reynolds JC et al., 2021).Osteoporosis
It shows promising potential in the management of osteoporosis, primarily due to its ability to regulate bone metabolism, reduce oxidative stress, and improve systemic metabolic health, all of which are key contributors to bone integrity.1. Promotion of Osteoblast Activity
MOTS-c has been shown to enhance osteoblast differentiation and function, the cells responsible for bone formation. By activating the AMPK pathway, MOTS-c supports energy homeostasis in osteoblasts, allowing them to better synthesize bone matrix and mineralize it effectively (Zhao J et al., 2021).- AMPK activation also leads to upregulation of RUNX2, a transcription factor essential for osteoblast development.
- It promotes bone anabolic pathways, counteracting the bone-resorptive effects seen in osteoporosis.
2. Inhibition of Osteoclastogenesis
It may inhibit osteoclast differentiation indirectly by reducing systemic inflammation and oxidative stress, which are known stimulators of osteoclast formation. Osteoclasts break down bone tissue; thus, MOTS-c’s ability to suppress excessive bone resorption contributes to preserving bone mass.3. Reduction of Oxidative Stress and Mitochondrial Dysfunction
Oxidative stress is a major factor in age-related bone loss. MOTS-c has antioxidant properties and maintains mitochondrial function, both of which help preserve osteocyte viability and bone homeostasis (Kim KH et al., 2018).- Improved mitochondrial signaling supports the survival of bone-forming cells under stress conditions.
- It also reduces ROS (reactive oxygen species) levels that can trigger bone resorption.
4. Metabolic Health and Bone Cross-talk
By improving insulin sensitivity, reducing fat mass, and enhancing muscle function, MOTS-c indirectly contributes to bone health. There is increasing evidence that metabolic diseases like obesity and diabetes worsen osteoporosis risk; thus, MOTS-c’s broad metabolic benefits support skeletal protection (Lee C et al., 2015).Role in cardiometabolic health
MOTS-c (Mitochondrial ORF of the 12S rRNA-c) plays a protective and regulatory role in cardiovascular health through multiple mechanisms involving metabolic regulation, oxidative stress reduction, and inflammation control. Recent evidence suggests that MOTS-c may be a promising therapeutic target for preventing and treating cardiovascular diseases (CVD), especially in aging and metabolic syndromes.1. Protection Against Ischemic Injury
It has been shown to reduce myocardial damage in experimental models of ischemia-reperfusion injury. It does so by:- Activating AMPK, which enhances energy production during oxygen deprivation.
- Improving mitochondrial function and ATP availability in cardiomyocytes.
- Suppressing cell death pathways (e.g., apoptosis) associated with reperfusion injury (Zhang Y et al., 2021).
2. Reduction of Oxidative Stress
Cardiac tissues are highly sensitive to oxidative stress, a key factor in heart failure and atherosclerosis. MOTS-c:- Decreases ROS (reactive oxygen species) levels.
- Enhances expression of antioxidant genes via NRF2 activation.
- Helps maintain redox homeostasis in cardiomyocytes (Kim KH et al., 2018).
3. Anti-inflammatory Effects
Chronic inflammation is a known contributor to vascular dysfunction and atherosclerosis. MOTS-c has been shown to:- Reduce circulating pro-inflammatory cytokines (e.g., TNF-α, IL-6).
- Inhibit NF-κB signaling, a central pathway in vascular inflammation (Lee C et al., 2015).
- Protect endothelial cells from inflammatory damage, improving vascular function.
4. Improvement in Metabolic Cardiac Health
Metabolic diseases such as obesity and insulin resistance contribute to cardiac dysfunction. By improving insulin sensitivity and reducing lipid accumulation, MOTS-c helps:- Prevent cardiac steatosis (fat deposition in the heart).
- Enhance cardiac glucose utilization, especially under stress or in diabetic hearts.
- Support cardiac contractility and output through efficient energy metabolism (Reynolds JC et al., 2021).
5. Support of Vascular Function and Blood Pressure Regulation
MOTS-c may have indirect benefits on blood pressure and vascular tone by:- Enhancing endothelial nitric oxide synthase (eNOS) activity.
- Promoting vasodilation through AMPK-mediated pathways.
- Preserving vascular compliance in aging or diseased vessels (Lu H et al., 2023)
References
- Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–54.
- Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun. 2021;12(1):470.
- Lu H, Buchan RJ, Cook SA. Mitochondrial peptides: a new class of signaling molecules in metabolism. Cell Metab. 2019;30(4):768–80.
- Kim KH, Son JM, Benayoun BA, Lee C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018;28(4):516–24..
- https://doi.org/10.1016/j.cmet.2015.02.009
- Zhang Y, Wang Y, Chen X, Wang D, Zhang D. MOTS-c protects against myocardial ischemia/reperfusion injury via activation of AMPK and reduction of oxidative stress. Int J Mol Sci. 2021;22(5):2453.
- Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28(4):516–24.
- Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–54.
- Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun. 2021;12(1):470.
40mg/ml solution provided in a 4ml vial.
“The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance Mitochondria are known to be functional organelles, but their role as a signaling unit is increasingly being appreciated.
The identification of a short open reading frame (sORF) in the mitochondrial DNA (mtDNA) that encodes a signaling peptide, humanin, suggests the possible existence of additional sORFs in the mtDNA. Here we report a sORF within the mitochondrial 125 RNA encoding a 16-amino-acid peptide named MOTS-c (mitochondrial open reading frame of the 12S rRNA-c) homeostasis. Its primary target organ appears to be the skeletal muscle, and its cellular actions inhibit the folate cycle and its tethered de novo purine biosynthesis, leading to AMPK activation. MOTS-c treatment in mice prevented age-dependent and high-fat-diet-induced insulin resistance, as well as diet-induced obesity.
These results suggest that I mitochondria may actively regulate metabolic homeostasis at the cellular and organismal level via peptides encoded within their genome. that regulates insulin sensitivity and metabolic Lee, Changhan & Zeng, Jennifer & G. Drew, Brian & Sallam, Tamer & Martin-Montalvo, Alejandro & Wan, Junxiang & Kim, Su-Jeong & Mehta, Hemal & Hevener, Andrea & Cabo, Rafael & Cohen, Pinchas. (2015). The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Keduces Obesity and Insulin Kesistance. Cell metabolism. 21. 443-494.”
The identification of a short open reading frame (sORF) in the mitochondrial DNA (mtDNA) that encodes a signaling peptide, humanin, suggests the possible existence of additional sORFs in the mtDNA. Here we report a sORF within the mitochondrial 125 RNA encoding a 16-amino-acid peptide named MOTS-c (mitochondrial open reading frame of the 12S rRNA-c) homeostasis. Its primary target organ appears to be the skeletal muscle, and its cellular actions inhibit the folate cycle and its tethered de novo purine biosynthesis, leading to AMPK activation. MOTS-c treatment in mice prevented age-dependent and high-fat-diet-induced insulin resistance, as well as diet-induced obesity.
These results suggest that I mitochondria may actively regulate metabolic homeostasis at the cellular and organismal level via peptides encoded within their genome. that regulates insulin sensitivity and metabolic Lee, Changhan & Zeng, Jennifer & G. Drew, Brian & Sallam, Tamer & Martin-Montalvo, Alejandro & Wan, Junxiang & Kim, Su-Jeong & Mehta, Hemal & Hevener, Andrea & Cabo, Rafael & Cohen, Pinchas. (2015). The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Keduces Obesity and Insulin Kesistance. Cell metabolism. 21. 443-494.”